

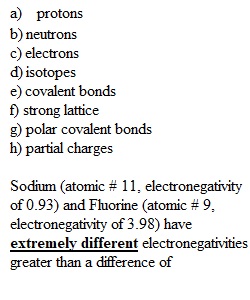
Q Critical thinking 1 25 points 1. Fill in the blanks to the following concept map (4 pts.): 2. Sodium (atomic # 11, electronegativity of 0.93) and Fluorine (atomic # 9, electronegativity of 3.98) have extremely different electronegativities greater than a difference of 2. Based on this information, how do you think Sodium and Fluorine would interact to form the compound Sodium Fluoride? (6 points) a. How many electrons are found in the valence shell of Fluorine prior to bonding? b. How many electrons are found in the valence shell of Sodium prior to bonding? c. How many electron shells are present in Fluorine prior to bonding?
Q d. What type of bond forms between Sodium and Fluorine (2 points)?
View Related Questions